Medical Certifications
Specific grades of Master Bond products fully meet the USP Class VI and ISO 10993-5 requirements. Medical grade adhesives, sealants, coatings and potting compounds need to be non-toxic and show compatibility with blood and body fluids. Additionally, they must be biologically inert. U.S. Pharmacopeia (USP) Class VI and ISO 10993 (Cytotoxicity) are two standard tests used to determine their suitability.

USP Class VI

ISO 10993-5 for Cytotoxicity
Master Bond's Biocompatible Adhesives
Master Bond develops and manufactures adhesives of different chemistries for medical device applications, including epoxies, silicones, UV & LED curable systems, urethane-modified epoxies, cyanoacrylates and specialty solvent based systems.
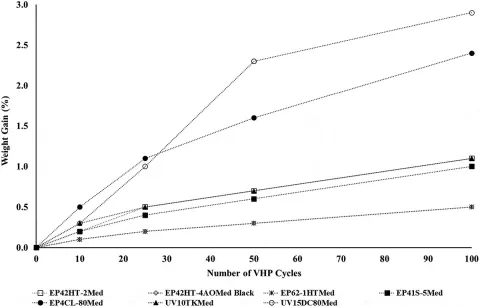
These compounds can simplify and enhance the performance of many critical reusable and disposable medical devices. Specific formulations offer high physical strength properties and outstanding chemical resistance even when applied on dissimilar or difficult to bond materials. Additionally, selected products retain their desirable performance upon exposure to various sterilization procedures including autoclaving, radiation, ethylene oxide as well as cold sterilants.
Latest Developments

Find the Right Adhesive for Your Application
With thousands of formulations, our expert engineers provide personalized guidance to ensure you select the ideal material for your specific needs.
Prefer to browse on your own first? Explore our Product Selector.